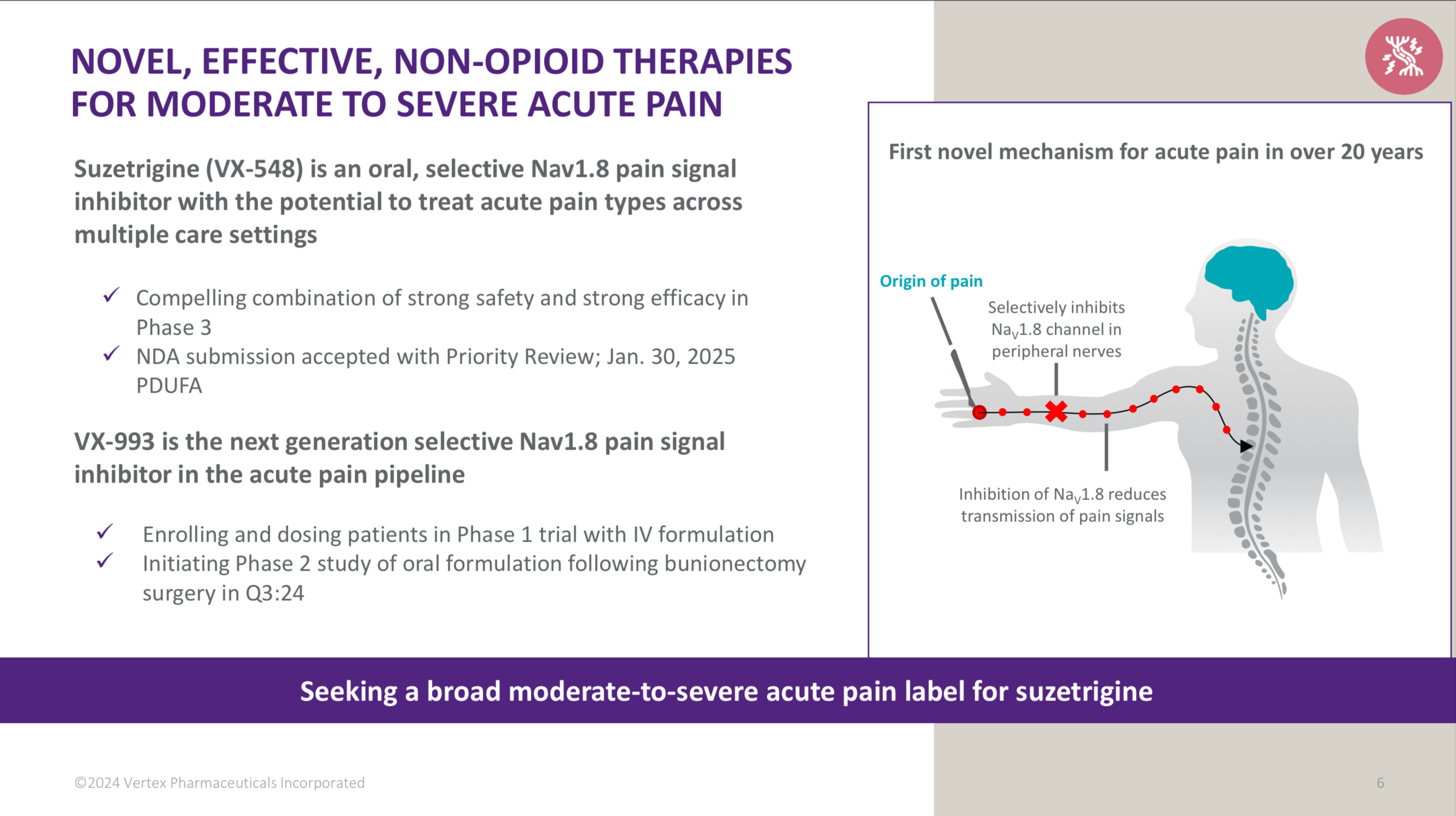
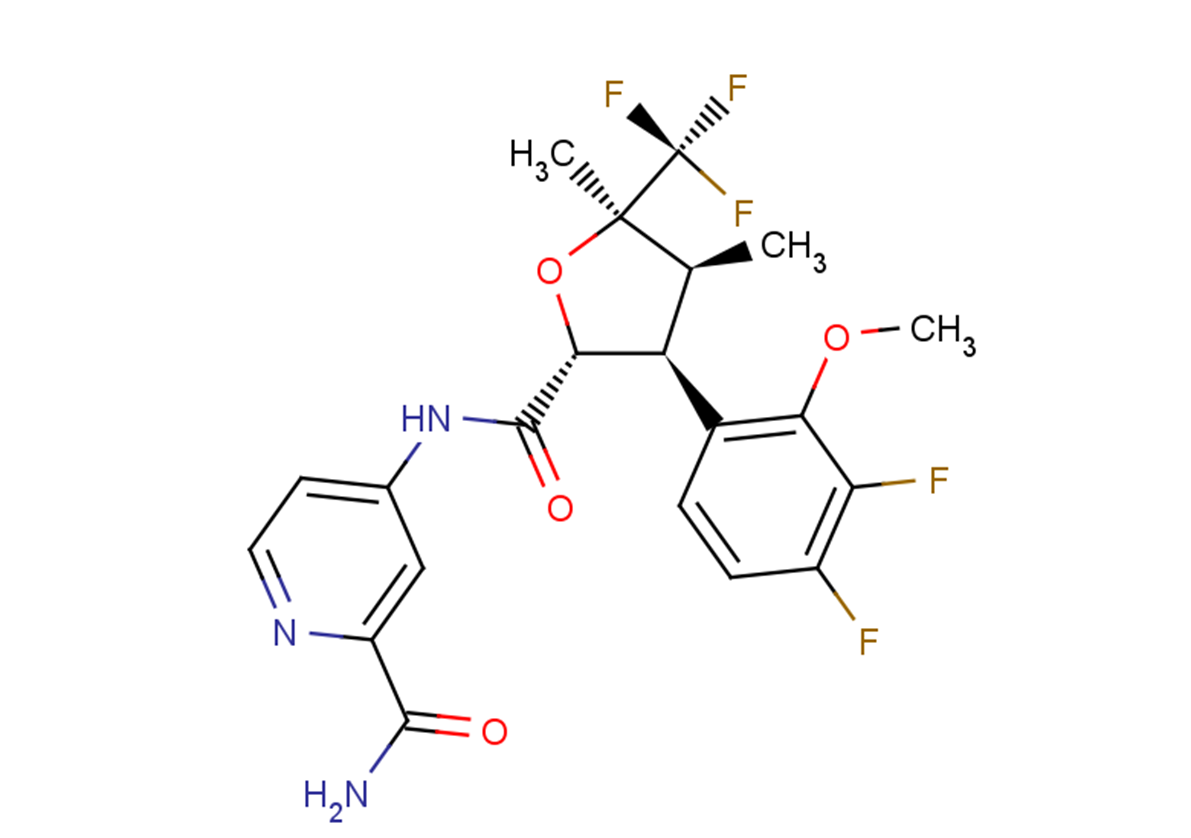
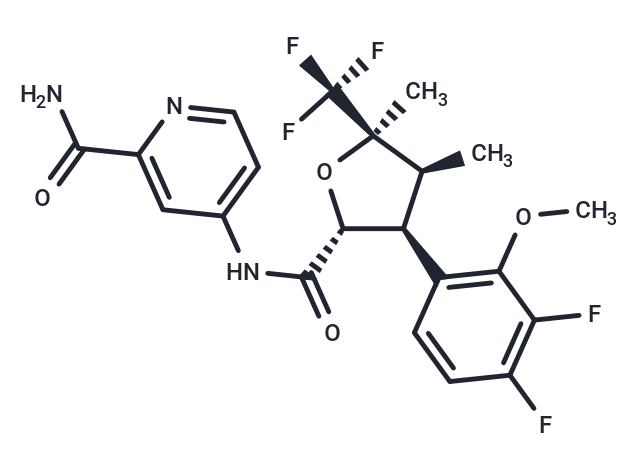
Suzetrigine, sold under the brand name Journavx, is a medication used for the management of pain. It is a non-opioid, small-molecule analgesic that works as a selective inhibitor of Nav1.8-dependent pain-signaling pathways in the peripheral nervous system, avoiding the addictive potential of opioids. Suzetrigine is taken by mouth.
The most common adverse reactions include itching, muscle spasms, increased blood level of creatine kinase, and rash.
It was developed by Vertex Pharmaceuticals, and was approved for medical use in the United States in January 2025. Suzetrigine is the first medication to be approved by the US Food and Drug Administration (FDA) in this new class of pain management medicines.
Medical uses
Suzetrigine is indicated for the treatment of moderate to severe acute pain in adults.
Efficacy
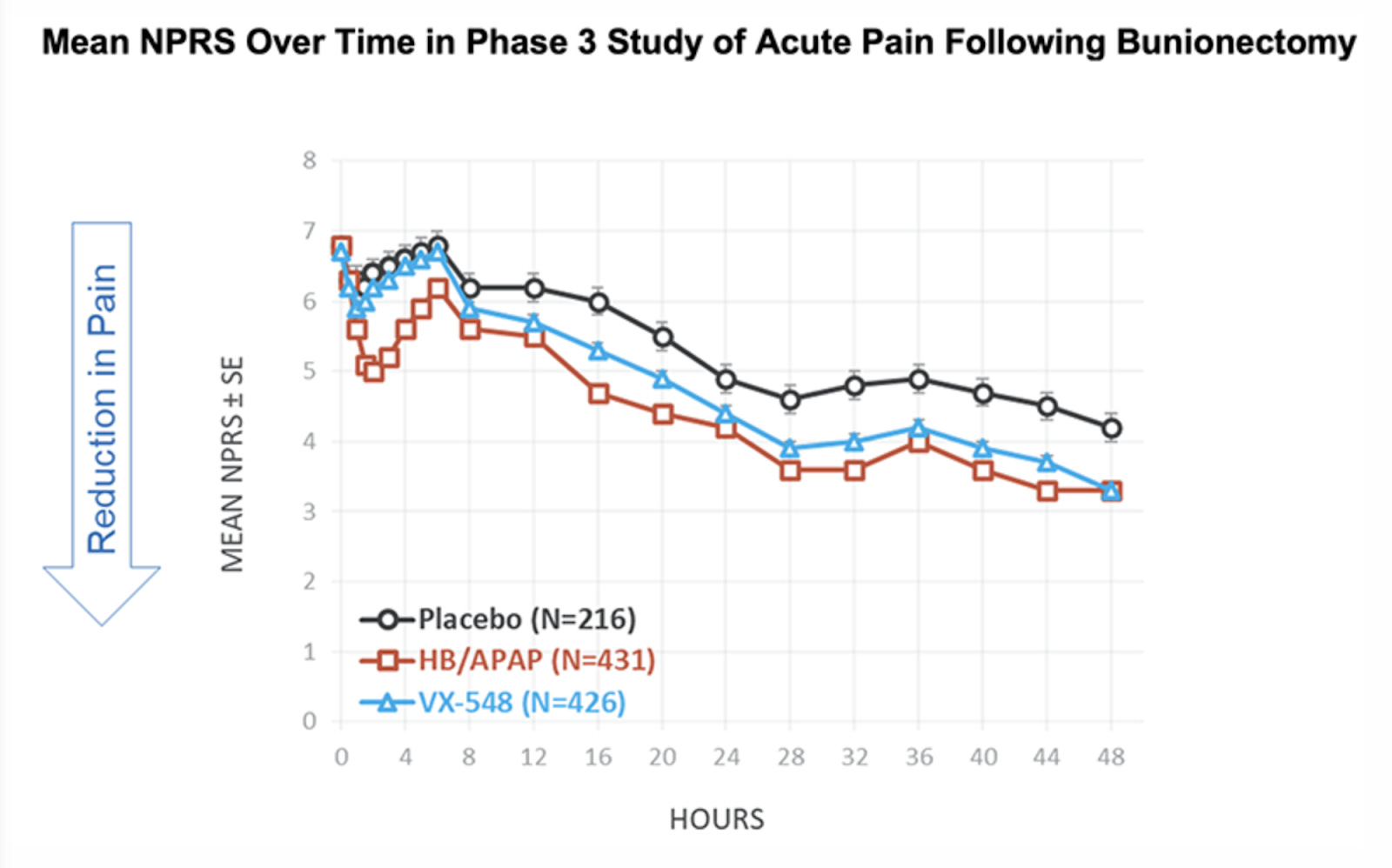
When people used suzetrigine in clinical studies conducted through 2024, there was a reduction in pain typically from seven to four on the standard numerical scale used to rate pain. Suzetrigine provided pain relief equal to a combination of hydrocodone and paracetamol (acetaminophen) (5 mg of hydrocodone bitartrate and 325 mg of acetaminophen).
Suzetrigine suppresses pain at the same level as an opioid, but without the risks of addiction, sedation, or overdose. An alternative to opioids, it is the first pain medication to be approved by the Food and Drug Administration in two decades.
The efficacy of suzetrigine was evaluated in two randomized, double-blind, placebo- and active-controlled trials of acute surgical pain, one following abdominoplasty and the other following bunionectomy. Both trials found that suzetrigine reduced pain more effectively than a placebo.
Contraindications
Concomitant use of suzetrigine with strong CYP3A inhibitors is contraindicated.
Adverse effects
Common adverse effects of suzetrigine may include itching, rash, muscle spasms, and increased levels of creatine kinase. Mild side effects may include nausea, constipation, headache, and dizziness. As of 2024, long-term safety and side effects remain undetermined.
In preliminary research, suzetrigine had no serious neurological, behavioral, or cardiovascular effects.
Interactions
Consuming grapefruit while using suzetrigine may cause an adverse grapefruit–drug interaction.
Mechanism of action
Suzetrigine operates on peripheral nerves, avoiding the addictive potential of opioids which affect the central nervous system. Unlike opioid medications, which reduce pain signals in the brain, suzetrigine works by closing sodium channels in peripheral nerves, inhibiting pain-signaling nerves from transmitting painful sensations to the brain.
In pharmacological studies, suzetrigine selectively inhibited Nav1.8 channels, but not other voltage-gated sodium channels, and bound to a unique site on these sodium channels with a novel allosteric mechanism, by binding to the channel's second voltage sensing domain, thereby stabilizing the closed state, causing tonic inhibition. It exerts its action on dorsal root ganglion.
History
Vertex Pharmaceuticals announced in January 2024 that suzetrigine had successfully met several endpoints in its Phase III clinical trials. The company announced in July 2024 that the FDA had accepted a new drug application for suzetrigine. The FDA granted the application for suzetrigine priority review, fast track, and breakthrough therapy designations. In January 2025, the FDA granted approval of Journavx to Vertex Pharmaceuticals.
Society and culture
Legal status
Suzetrigine was approved for medical use in the United States in January 2025.
Names
Suzetrigine is the international nonproprietary name.
Suzetrigine is sold under the brand name Journavx.
References
Further reading
- Oliver, Brian; Devitt, Catherine; Park, Grace; Razak, Alina; Liu, Sun Mei; Bergese, Sergio D. (2025). "Drugs in Development to Manage Acute Pain". Drugs. 85 (1): 11–19. doi:10.1007/s40265-024-02118-0. PMID 39560856.
External links
- "Suzetrigine (Code C199115)". NCI Thesaurus.
- Clinical trial number NCT05661734 for "A Single-arm Study to Evaluate Safety and Effectiveness of VX-548 for Acute Pain" at ClinicalTrials.gov
- Clinical trial number NCT05558410 for "Evaluation of Efficacy and Safety of VX-548 for Acute Pain After an Abdominoplasty" at ClinicalTrials.gov